We are trusted by many global satisfied users, Solisom Healthcare LLP is a global partner
for your next Fenofibrate API and intermediates need in the world’s largest Healthcare Industry.
Trusted
We are trusted by many global and local Pharmaceutical manufacturers by Quality, Stability and efficacy of our Fenofibrate API.
Economical
Our state of art R&D and team of techno -commercial expert bring you high quality Fenofibrate API at lowest cost possible.
Reliable
Our Quality Control Laboratory and Quality management system assure that, customer got best quality product.
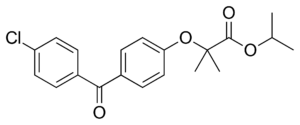
FENOFIBRATE - CAS No. : 49562-28-9
FENOFIBRATE (Micronized)
Category: Antilipemic Agent (Fibric Acid Derivative)
CAS No.: 49562-28-9
Chemical Name: Propan-2-yl 2-[4-(4-chlorobenzoyl)phenoxy]-2-methylpropanoate
Grades: IP / BP / USP / EP
DMF Status: Under Process
Solisom Healthcare LLP is one of the leading Fenofibrate API manufacturers, suppliers, and exporters based in Gujarat, INDIA. We provide micronized Fenofibrate in various pharmacopeial grades (IP, BP, USP, and EP) to support pharmaceutical manufacturers globally. Operating from our cGMP-compliant facility in Morbi, Gujarat, our team of experts ensures the production of high-purity Fenofibrate backed by comprehensive regulatory documentation and technical support.
Fenofibrate is a lipid-regulating agent used primarily to lower high cholesterol and triglyceride levels in the blood. As a prodrug of fenofibric acid, it helps increase the breakdown of lipids, reducing the risk of atherosclerosis and cardiovascular complications. Its stability and efficacy make it a staple in oral solid dosage formulations, particularly for managing hypercholesterolemia and mixed dyslipidemia.
Pharmaceutical Grades Offered
At Solisom Healthcare, we offer Fenofibrate in multiple pharmaceutical grades to meet global standards:
Fenofibrate IP (Indian Pharmacopoeia)
Fenofibrate BP (British Pharmacopoeia)
Fenofibrate EP (European Pharmacopoeia)
Fenofibrate USP (United States Pharmacopeia)
Each batch is synthesized to meet the rigorous purity and dissolution profiles required by these monographs, ensuring consistent therapeutic performance.
Quality Commitment
Quality is the cornerstone of our operations at Solisom Healthcare. Our Fenofibrate API is manufactured in state-of-the-art facilities adhering to international cGMP guidelines. From raw material sourcing to final micronization, we implement strict quality control measures to ensure a product that healthcare providers and patients can trust.
In Short: To source high-quality material from the leading Fenofibrate API manufacturers in India, contact us today.
Typical Applications
Oral Solid Dosages: Formulation of immediate-release and delayed-release tablets or capsules.
Micronized Formulations: Enhanced bioavailability versions for improved intestinal absorption.
Combination Therapies: Often co-formulated with statins for comprehensive lipid management.
Pharmaceutical R&D: Ideal for clinical trials, stability studies, and generic formulation development.
Package & Supply Options
Bulk Packaging: 5 kg, 10 kg, and 25 kg HDPE drums with dual LDPE liners (Customized packaging available on request).
Export-Ready: Full documentation including COA, MSDS, stability data, and technical dossiers.
Supply Capacity: Reliable contract manufacturing and bulk supply for domestic and international markets.
Quality Assurance & Regulatory Compliance
cGMP Manufactured: Produced in validated facilities located in Morbi, Gujarat.
Rigorous Testing: Every batch undergoes strict QC testing, including Assay (HPLC), related substances, particle size distribution (PSD), and residual solvents.
Regulatory Support: DMF support and analytical method validation are available for regulated markets.
Why Choose Solisom Healthcare for Fenofibrate?
Trusted Indian Supplier: Extensive experience in regulated exports and domestic distribution.
Superior Impurity Profile: High assay levels and low impurity limits for optimal safety.
Technical Expertise: Specialized in particle size optimization (micronization) for better solubility.
Efficient Logistics: Flexible packaging and timely international shipments.
Contact & Orders
Contact us today to discuss your specific requirements for particle size, pricing, or lead times. Our technical team is ready to assist with formulation guidance tailored to your target market.
